Equilibria of Solution Critical Point
Contact: DR. JAMES BAIRD
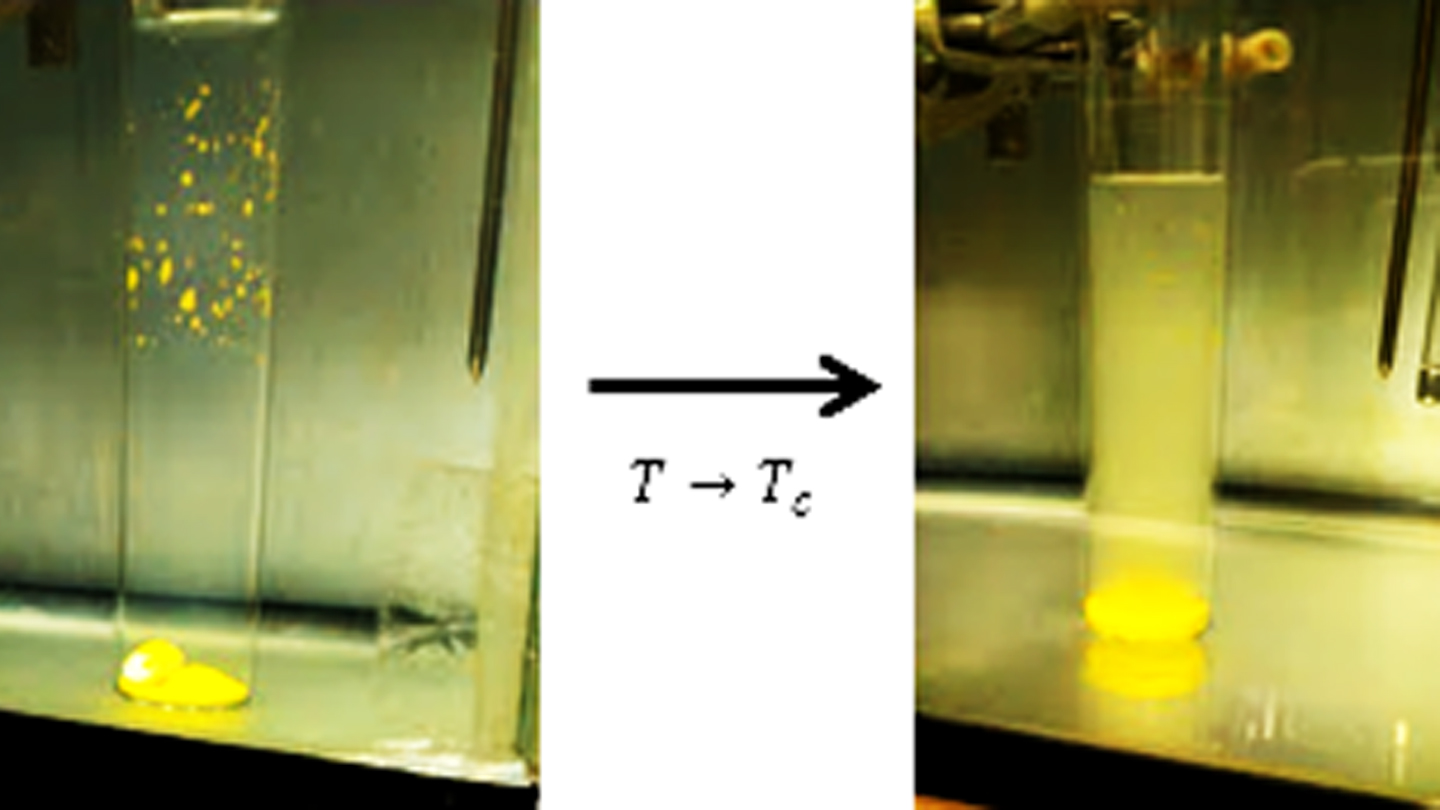
‘Critical phenomenon’ are changes in material properties that result from the continuous transformation of one state or phase of matter into another without the immediate appearance of a phase boundary. Such phenomena are ubiquitous, occurring in ferromagnetic materials, substitutional alloys, and binary liquid mixtures with a critical point of solution.
We have shown that the universal appearance of critical behavior in the physical properties of a binary liquid mixture can be extended to include the chemical properties. Studies so far have involved solubility, adsorption, and ion exchange. By exploiting the Gibbs phase rule and the principle of critical point universality, we have found that chemical critical effects are to be expected whenever temperature, pressure, and one composition variable are held fixed.
Synthesis of New Biomedical Polymers

Contact: DR. CARMEN SCHOLZ
Synthesis and characterization of biodegradable, biocompatible and metabolizable poly(amino acid)s for new polymers with applications in biomedicine. These polymers have been evaluated in our group for coatings of biomedical implants, gene delivery vehicles and protein mimics.
