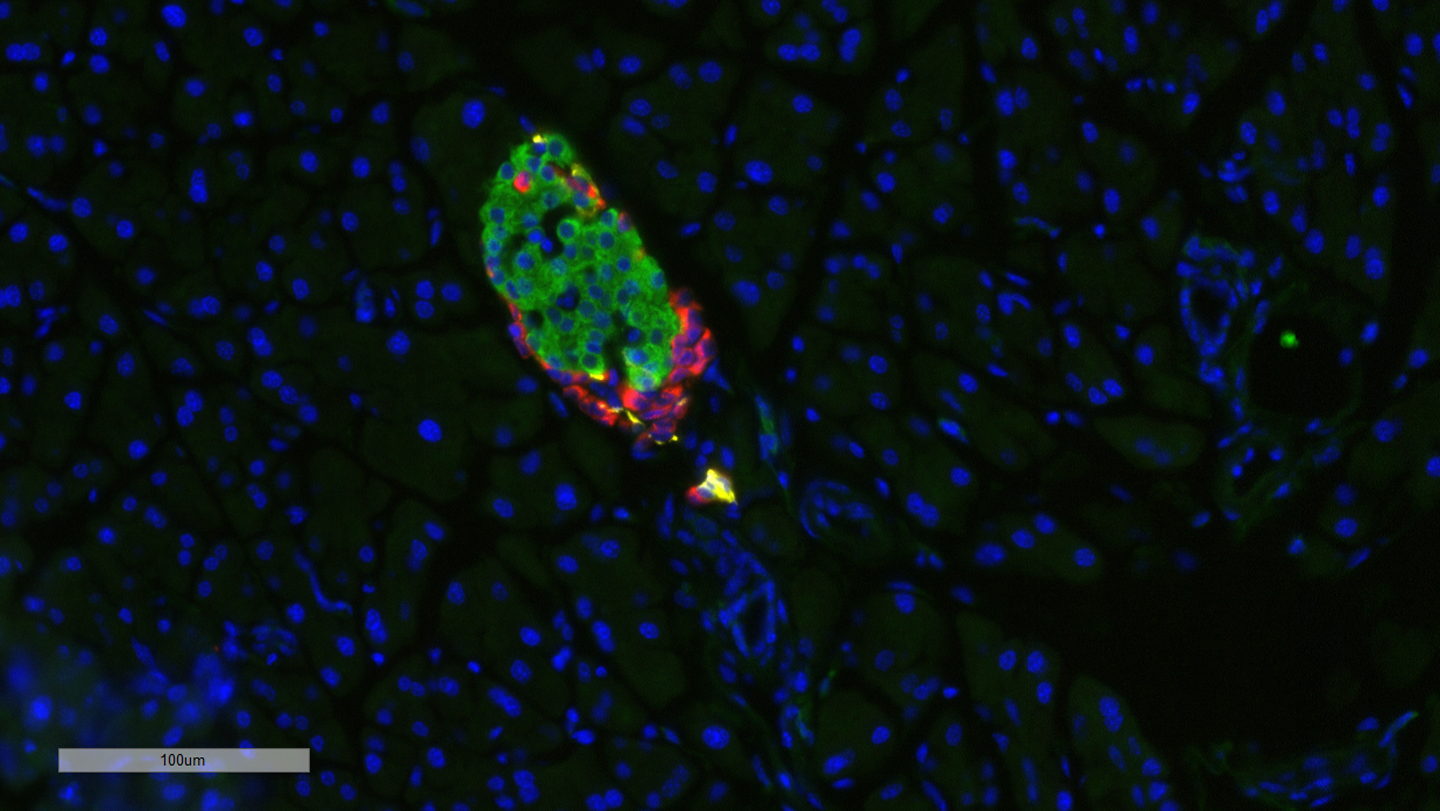
The image depicts morphologies of pancreatic islets. Sections were stained with antibodies to insulin (red), glucagon (yellow) and somatostatin (green), while the DNA was stained with DAPI (blue). DAPI is a blue fluorescent stain used to visualize cell nuclei, most notably within the pancreatic islets, which contain insulin-producing beta cells. By staining the cell nuclei, DAPI provides a reference point for analyzing changes in the size, shape and structure of the islets and individual cells in diabetic and healthy tissue.
Courtesy Ahmed Lawan
Dr. Ahmed Lawan, an assistant professor in the College of Science at The University of Alabama in Huntsville (UAH), a part of The University of Alabama System, has published a paper that demonstrates there is a potential gender component in the development of diabetes as it relates to the function of a particular enzyme. The study in the National Institutes of Health (NIH) National Library of Medicine has won a $50,000 NIH grant to examine the sex-specific role of Mitogen-Activated Protein Kinase Phosphatase (MKP-2) enzyme in diabetes.

Dr. Ahmed Lawan, an assistant professor in the College of Science at The University of Alabama in Huntsville.
Michael Mercier / UAH
“Previous studies in the literature showed that men are more likely to develop diabetes than women,” Dr. Lawan explains. “However, women are more likely to develop more complications compared with men. In this study we sought to investigate the role of MKP-2 in the development of diabetes and uncovered sex-specific differences with regard to the development of diabetes in MKP-2-deficient mice.”
MKP-2-deficient mice, known as MKP-2 knockout mice, are genetically modified mice that lack functional MKP-2, a protein involved in regulating immune and metabolic processes. By studying the metabolic differences between mice lacking the enzyme (knockout mice) and those with it (control mice), researchers gain insight into the function of MKP-2 and its potential as a sex-specific target for high blood sugar and beta cell dysfunction.
The effort is a follow-up to an initial characterization of the metabolic function of MKP-2, shown to be significant in diabetes research, because its upregulation is linked to the development of insulin resistance, obesity and fatty liver disease. In diabetes research, upregulation refers to the increase in the production or number of specific molecules, such as enzymes, receptors, proteins or genes, that can either contribute to diabetes development or serve as possible new regulatory pathways.
“Currently, there is a lack of medical recommendations regarding sex-specific preventive measures and management of diabetes,” Dr. Lawan notes. “This is due to a lack of preclinical research done on both sexes, because most of the research has been focused on males. Depending on the stage of reproductive life, the sex difference in diabetes prevalence is reversed; that is, more men have the disease before puberty, while more women have it after menopause and in later life.”
MKP-2 deactivates signaling enzymes called mitogen-activated protein kinases by removing phosphate groups from them, critical for regulating the inflammatory response and controlling metabolic processes.
The development of type 2 diabetes (T2D) is largely dependent on the maintenance of pancreatic islet function and mass. Pancreatic islets play a critical role, because they are the site of insulin production, a hormone crucial for regulating blood glucose. Islet function is central to diabetes development, as defects in islet cells and their ability to secrete insulin are a major cause of the disease.
The new study investigates the role of MKP-2 in regulating glucose homeostasis, or the body's ability to maintain stable blood glucose levels despite various factors, like dietary intake, exercise and stress. Disruptions in glucose homeostasis are a hallmark of diabetes. Results show that female mice with MKP-2 deletion exhibit hyperglycemia and reduced pancreatic islet size.
Under type 2 conditions, MKP-2-deficient mice display enhanced pancreatic phosphorylation – the activation of specific cellular signaling pathways involving two types of enzymes – important for islet development and function. The research concludes that MKP-2 may have a gender-specific role in diabetes development, potentially benefiting postmenopausal prevention of T2D.
“More studies are needed to predict how MKP-2 may act in diabetes,” Dr. Lawan concludes. The initiative has been supported by UAH graduate students Nabin Ghimire, Morgan Welch, Cassandra Secunda and Alexis Fink.