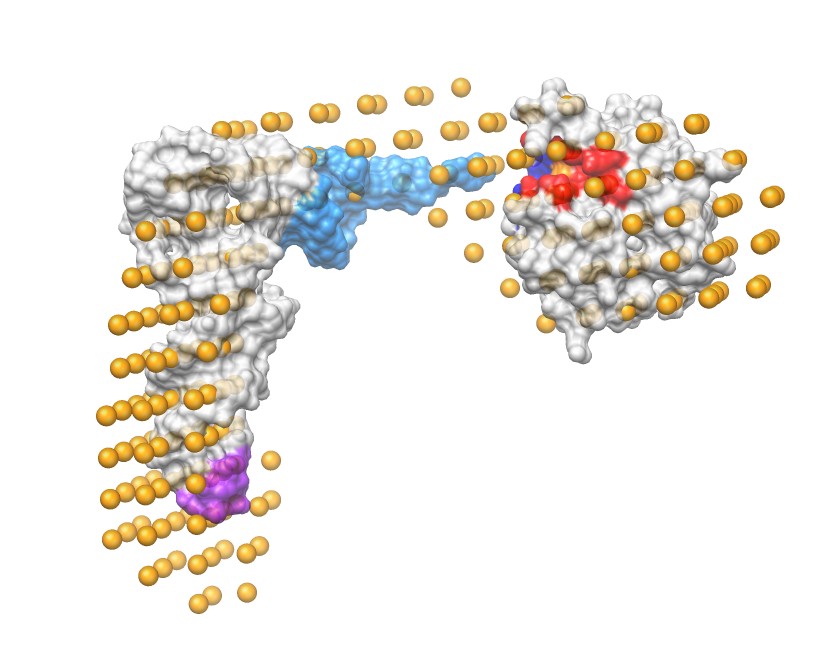
Peptidyl-tRNA are generated from stalled ribosomes. Peptidyl-tRNA hydrolases (Pths) are the enzymes essential for the removal of bound peptides from tRNA molecules. Peptidyl-tRNAs are toxic to cells and without Pth recycling of peptidyl-tRNA, cells die due to impaired translation initiation and slowed protein synthesis due to specific tRNA starvation. The essential activity of bacterial Pth, Pth1, makes it a high value drug target. Disrupting Pth1 activity leads to bacterial death and since no essential Pth1 homolog is found in humans, few side effects are expected from Pth1 inhibitors. We have solved the structure of the Pth:peptidyl-tRNA complex using small-angle neutron scattering and are currently adding high resolution information. We are screening natural products, including tropical cloudforest and aquatic fungal extracts for Pth1 inhibitors. Numerous extracts have been identified with anti-Pth activity and identification of the active compounds is underway. These diverse sources are expected to contain phytochemicals and secondary metabolites with novel structural motifs and novel mechanisms of bioactivity, hopefully contributing to development of the next generation of antibiotics.
Crystallization
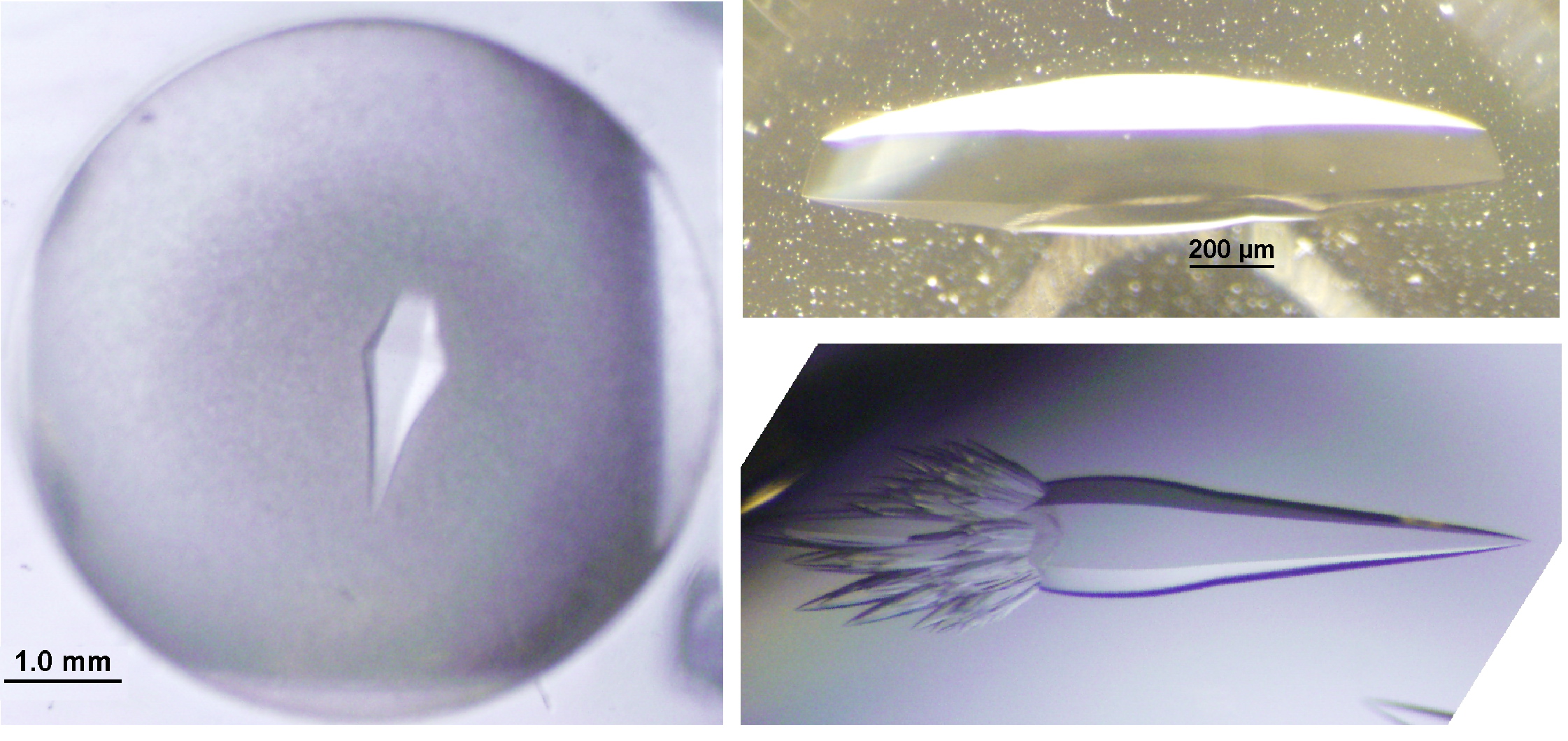
X-ray Diffraction Structures
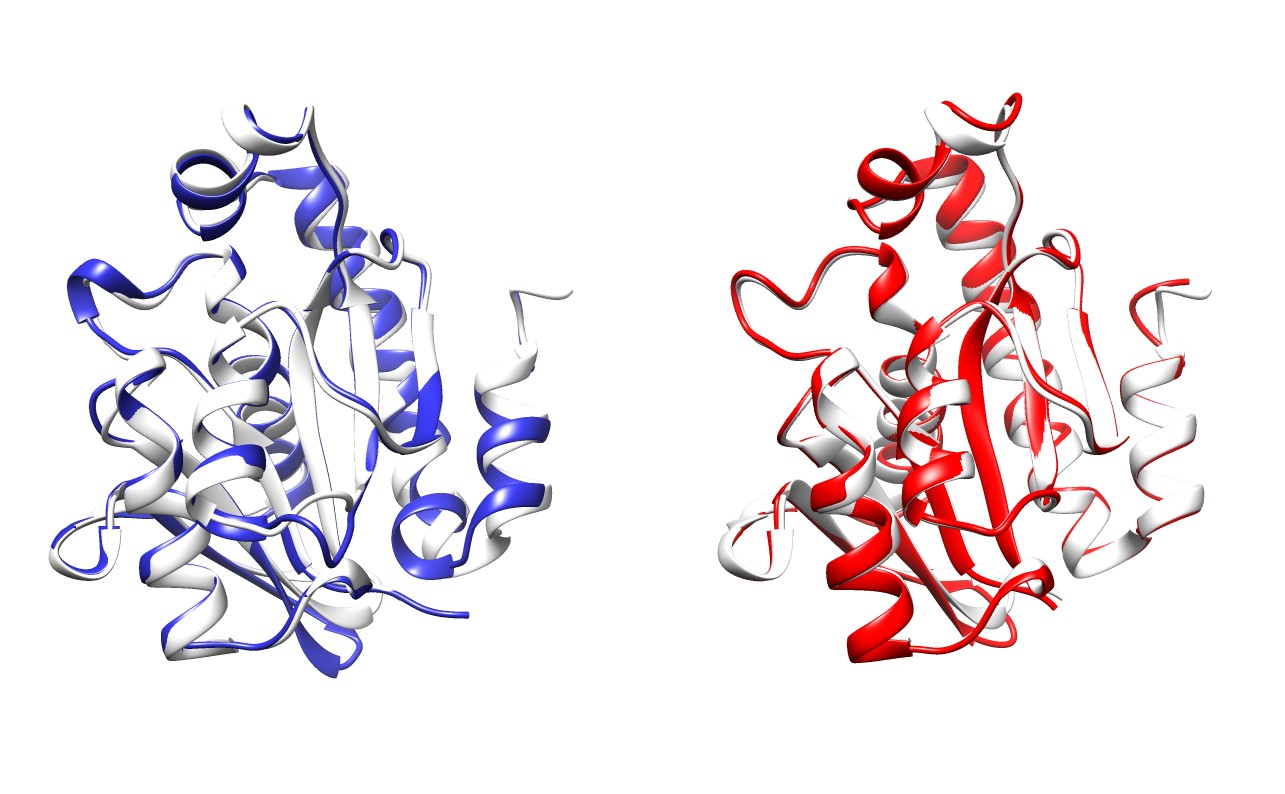
Overlay of Pth1 from P. aeruginosa (blue) and S. typhimurium (red) onto E. coli (white).
NMR Resonance Assignment
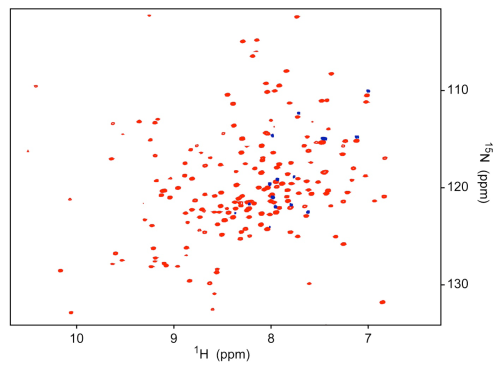 |
15N-TROSY HSQC at 800 MHz of Pth (red) with15N-phenylalanine residue specifically labeled resonances offset (blue). |
Inhibitor Screening
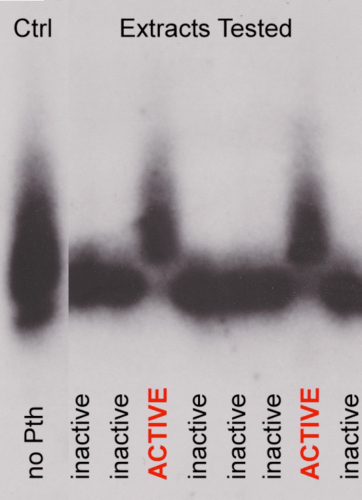 |
Northern blot of peptidyl-tRNA migration.
|
Inhibitor Identification
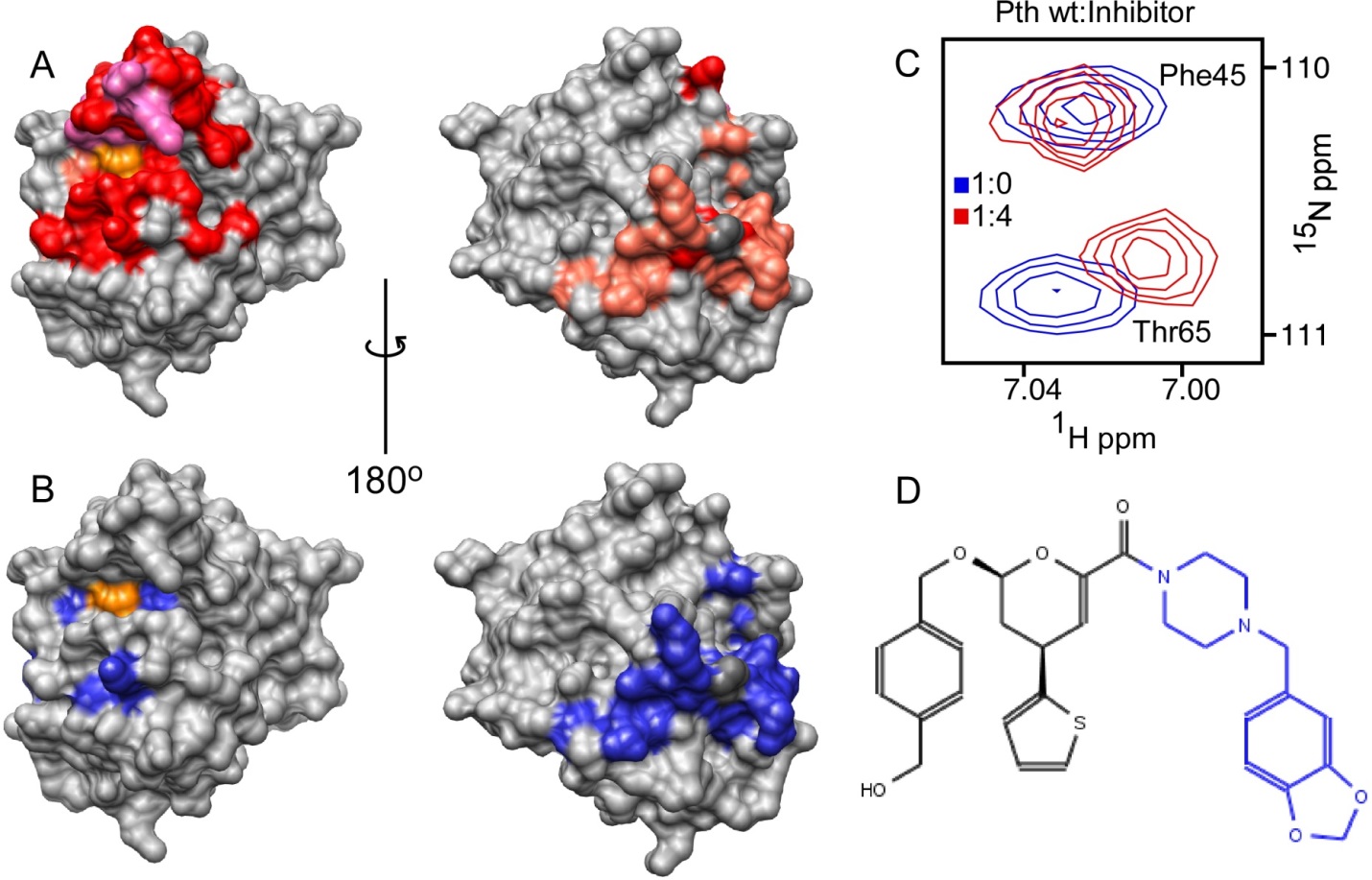
Interaction of E. coli Pth1 with a small molecule inhibitor (synthetic molecule from collaborator’s library). In A), the chemical shift changes upon titration of the entire molecule (shown in D) are indicated. In B), the changes from just the blue portion of the molecule (piperonylpiperazine). In C), a zoomed section of the spectra in which Phe45 shows no changes, whereas Thr65 is perturbed by binding. D) The structure of the synthetic inhibitor used for titration.
Small Angle Neutron Scattering